-40%
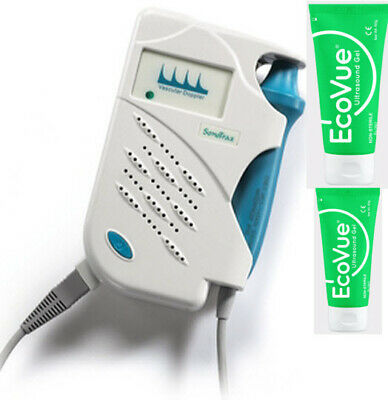
Ankle Brachial Vascular Doppler cum Digital Biothesiometer VPT Model VOBRODOP
$ 1188
- Description
- Size Guide
Description
Ankle Brachial Vascular Doppler cum Digital Biothesiometer VPT Model VOBRODOPIndia’s first innovative design that has combined Digital Biothesiometer for the detection of loss of vibration perception for assessing diabetic neuropathy and a Vascular Doppler for the measurement of Ankle Brachial Index (ABI). Computer connectivity is a built-in facility and reports are generated by the software supplied.
The Ankle Brachial Index (ABI) is a measure of the severity of atherosclerosis in the legs but is also an independent indicator of the risk of subsequent atherothrombotic events elsewhere in the vascular system. The ABI may be used as a risk marker both in the general population free of clinical CVD and in patients with established CVD.
Vibration Perception Threshold (VPT) has been shown to be strongly associated with foot ulceration. VPT determination by using a Digital Biothesiometer has been used to identify peripheral sensory neuropathy and subjects at risk of foot. Digital Biothesiometry helps us quantitate the threshold and monitor progressive changes or trends on following up testing.
Sensory neuropathy increases the risk of foot ulcerations by seven folds and peripheral arterial disease (PAD) by three folds in people with diabetes. Peripheral neuropathy is the major causal factors in the development of foot ulcerations among diabetic subjects.
Early detection helps better prevention. 50% of all non-traumatic amputations occur in diabetes who is a high risk group.
Features:
Doppler (C.W) with 8MHz Uni-Directional probe
Ankle Brachial Index
Doppler Velocity Waveform with ABI Indices
0 to 50 Volts Vibration linear output
Computer connectivity enabled
Software for data transfer, storage and patient report
Software supports all formats of Windows (Xp to Win10) OS
Standards matching International standards
-------------------------------------------------------------------------------------------
Custom Policy
Import duties, taxes and charges at customer locations are not included in the item price.
These charges are the buyer's responsibility. Please check with your local customs authority to
determine these additional costs prior to bidding or buyin
g.
COMPANY INFORMATION
We m/s DIABETIK FOOT CARE INDIA PVT LIMITED are an ISO 13485:2016 certified company engaged in manufacturing, distributing and exporting a comprehensive range of diagnostic and therapeutic products for the management of diabetes and its complication.
We have more than 27 years experience in manufacture and export of Medical Devices.
Why Us
We have created a benchmark in the industry and following are the features that have won for us the critical appreciation of our clients:
High quality
Innovation
Timely delivery
After sale support
Varied range of products
Convenient payment mode
Customer friendly
Experience in International market
Continuous improvement
Infrastructure
We have employed state of the art infrastructure that is built on the most sophisticated designing and manufacturing capabilities. Our production facility is installed with advanced software and machines. Our infrastructure also consists of a well maintained packaging and warehousing unit. We make sure that all the products are packaged, stored and transferred under quality control environment so that they reach our esteemed customers in perfect conditions. Most of our prime products are tested for electrical safety, EMI and EMC in a NABL accredited test lab in India
Clientele
Our clientele list are growing steadily and we have supplied more than 7000 of our products to 36 countries. Most of the leading Institutions in India treating diabetes are our clients. Our products are exported to the following countries either directly or through our International distributors - Australia, Bangladesh, Bolivia, Bulgaria, China, Cyprus, Czech, Denmark, Egypt, El-Salvador, Haiti, India, Indonesia, Israel, Kenya, Kuwait, Macedonia, Malaysia, Magnolia, Mauritius, Myanmar, Nepal, Nigeria, Oman, Pakistan, Palestine, Philippines, Saudi Arabia, Singapore, South Africa, Sri Lanka, Taiwan, Tanzania, Thailand, UAE, USA and Yemen.
Disclaimer:-
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."